Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good morning. We’ve got more news out of DC today – let’s get into it.
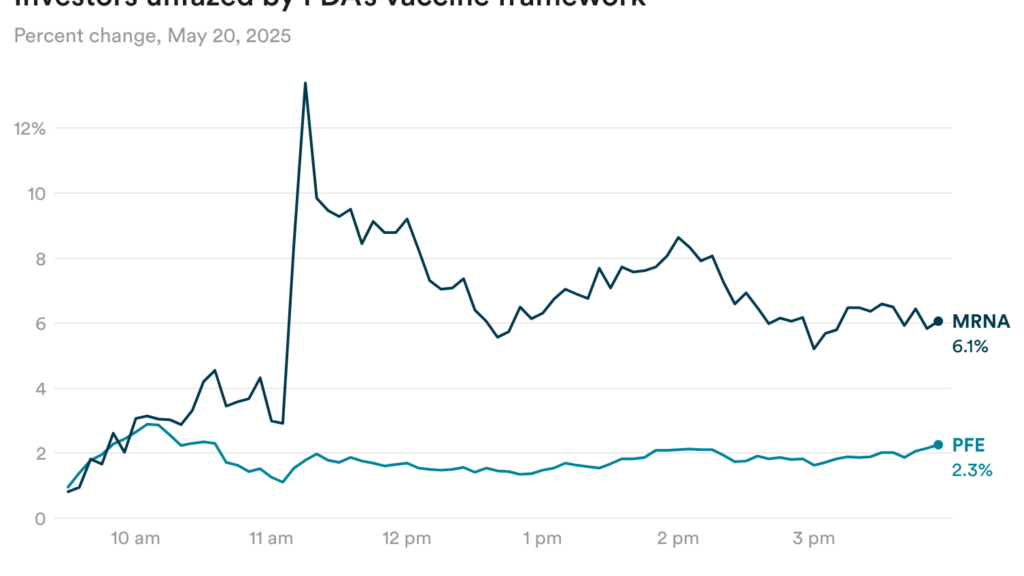
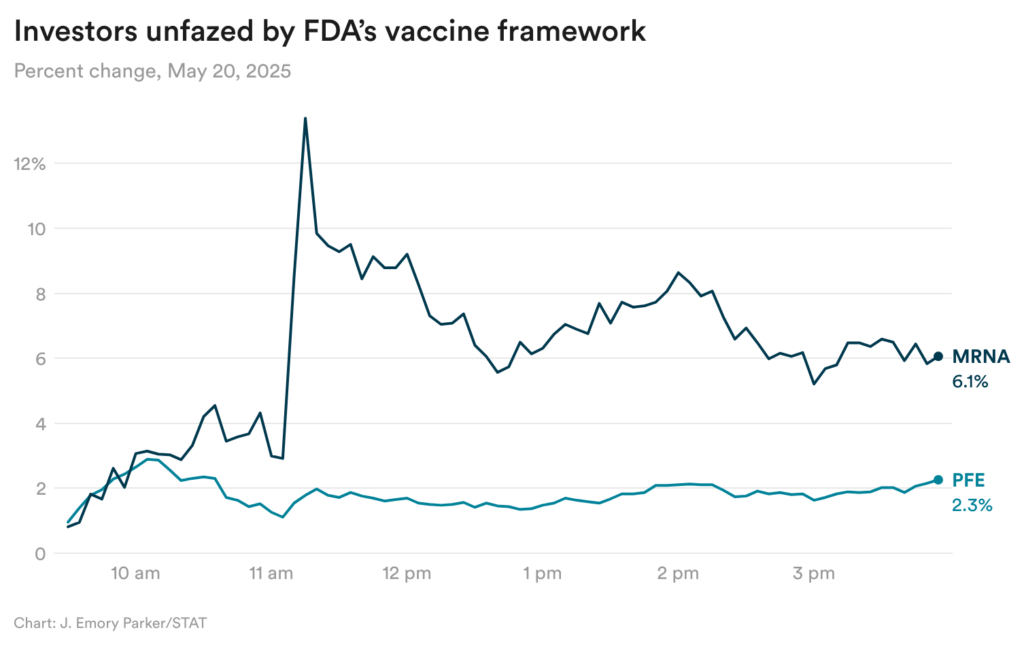
Investors unfazed by FDA’s Covid vaccine framework
The FDA announced yesterday that it will limit access to Covid-19 vaccines to people 65 years of age and older and others who are at high risk of becoming seriously ill. It will also require manufacturers to conduct clinical trials to show whether the vaccines benefit healthy younger adults and children.
The framework, published in the New England Journal of Medicine, comes after leaders at the agency signaled a shift in thinking about Covid vaccines.
Investors didn’t seem too concerned by the framework, though, as they were likely expecting a much more restrictive proposal. Shares of Moderna rose 6% yesterday, while shares of Pfizer were up 2% (though Pfizer’s stock was also affected by separate oncology news yesterday.)
In a note, Jefferies analysts wrote that that the framework won’t likely affect Pfizer’s vaccine sales much, since most of the revenue comes from the 65-and-older patient population.
Meanwhile, TD Cowen analysts wrote that the new framework is favorable for Moderna’s next-generation Covid vaccine, which is due for an FDA decision by the end of this month. Moderna has Phase 3 efficacy data for its vaccine that positions it well for approval in a broad population, giving it a competitive edge over Novavax’s recently approved vaccine, the analysts wrote.
The framework is also affecting Moderna’s strategy with other candidates. The company this morning said it’s withdrawn its FDA application for a flu/Covid-19 combination vaccine and plans to resubmit it later this year after it gets efficacy data from an ongoing Phase 3 trial. Moderna had already signaled this in earlier guidance.
Some more details on Trump’s drug pricing plan
The Trump administration yesterday shared more details of its plan to push pharmaceutical companies to lower their U.S. drug prices to be in line with prices in other countries.
Specifically, it’s telling companies to set their U.S. prices at the lowest level offered in countries that are part of the Organization for Economic Co-operation and Development and that have a GDP per capita that’s at least 60% of the U.S.’ (Countries that appear to meet those criteria include Germany, France, and the U.K.)
The pricing targets will apply to brand-name drugs that don’t face competition from generics or biosimilars. The announcement didn’t include a timeline for action, but said officials would highlight commitments by companies to lower their prices “in the coming weeks.”
There are still plenty of unanswered questions, though, such as: What are the consequences for companies that don’t lower their prices? Which prices (such as list or net) are the companies being asked to lower?
Read more from STAT’s Daniel Payne.
How much of an AI drug is actually made ‘from scratch’?
From my colleague Brittany Trang: Last week, AI drug development startup Absci announced that its first candidate, ABS-101 for inflammatory bowel disease, has entered the clinic. Though the company doesn’t say so in its press release, media coverage claims that Absci designed the antibody drug “from scratch” with AI.
Earlier this year, we examined these kinds of broad claims made by Absci and another AI drug development company from Flagship called Generate:Biomedicines, and we found that they were greatly exaggerated. At that time, Absci hadn’t shared many specific details about ABS-101.
We now have some more information. A newly released patent application for ABS-101 shows that half of the antibody derives from AI.
Where did the rest of the molecule come from? The company did not respond to a request from STAT, but Absci CEO Sean McClain has previously said that the company studied other TL1A assets from Merck and Roche to figure out what it wanted its model to do.
“I think this is a great use case from an IP standpoint — how could we use the AI to get outside of the existing IP from the competitors but then carve out new IP landscape for our own, which we have successfully done with this program?” McClain previously said at a 2024 JPM conference.
A version of this item also ran in AI Prognosis, STAT’s subscriber-only newsletter about AI in health and medicine written by Brittany Trang. Sign up here to get to the latest developments on AI in health care and to read more analysis that aims to separate hype from reality.